


The treatment of Parkinson’s disease is problematic. While it is defined primarily as a disease of compromised motor function there are other features of this disorder that grossly jeopardize quality of life. Primary, non-motor symptoms commonly reported include insomnia, depression, anxiety and nocturnal movement with dementia and cognitive deterioration observed at various times before or after the onset of the disease. When treatment is focused on reducing tremor, improving bradykinaesia and alleviating rigidity, primary non-motor symptoms are considered secondary in maintaining quality of life. In addition, prolonged treatment with drugs that replace dopamine exacerbate many of these symptoms and even produce additional side effects including psychosis and involuntary movement. For this reason it is commonly maintained that the treatment becomes worse than the disease itself. Some of the problems commonly observed in the treatment of Parkinson’s disease with dopamine replacement therapy include the following:
The depression of Parkinson’s disease is frequently unrecognized, ignored or inappropriately treated. It is interesting how the definition of depression so closely mimics Parkinson’s disease that the two might be impossible to differentiate. For example, one definition of depression illustrates the similarities:
The differential diagnosis of depression within Parkinson’s disease would be essential to treat each effectively. This, however, is not always possible given how depression is so intricately woven within the fabric of Parkinson’s disease. It has been estimated that more than 90% of Parkinson’s patients suffer from depression and that it may be present in these patients for 2 or 3 decades prior to diagnosis. This is confounded further by the reports that the depression of Parkinson’s disease does not always respond well to various forms of anti-depressant medication while some, such as the SSRIs, may even insidiously worsen the condition. How the co-expression of depression might contribute to the expression of Parkinson’s disease is expressed in diagram 1. This is why improving depression by non-invasive means plays an increasingly important role in disease management and why the application of light therapy will be important.
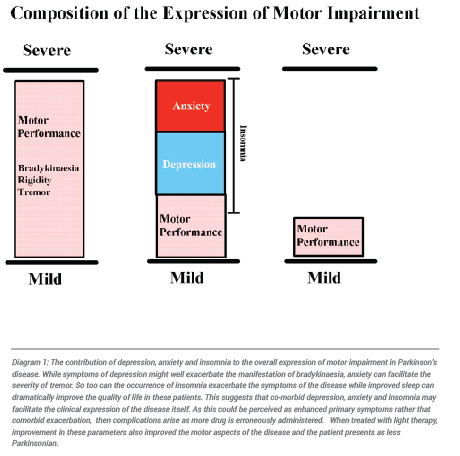
Anxiety is the major enemy of Parkinson’s disease. In the presence of a stressful situation not only do these patients routinely exhibit the physiological signs and symptoms of anxiety but in almost all cases their primary motor and non-motor symptoms are exaggerated. When this occurs the patient typically seeks additional medication to control what they perceive to be disease progression. With this adverse overdosing rapidly ensues. The patient is then inadvertently and unknowingly led into the ever increasing cycle of side effects and it is surmised that the subsequent adverse effects are a product of disease progression. In high anxiety patients attempts to reduce anxiety without drug intervention has proven extremely helpful in that drug interaction and unwanted side effects are avoided. The use of phototherapy in preventing “panic dosing” “has proven invaluable in many of these patients. This provides non-invasive relief from anxiety thereby improving the quality of life in these patients and reducing the need for increased dosing of dopamine replacement and anxiolytic drugs. In the longer term phototherapy has even been applied successfully within our clinic to reduce dyskinaesia and reduces or eliminates the need for brain surgery. To understand the contribution that anxiety can make to the expression and facilitation of Parkinsonian symptoms see Diagram 1 above.
Insomnia and, to a lesser degree, hypersomnia present a major problem for the patient with Parkinson’s disease. In either case the lack of adequate sleep and the increased sleep caused by many dopamine agonists results in fatigue, making daily existence a major burden. When sleep is repaired symptoms lessen and patients are free to pursue a more normal lifestyle. As is the case with depression and anxiety not only do basic motor skills improve but it is not uncommon that an improvement in depression and anxiety can often be observed when the sleep cycle improves returns to normal.
These secondary features of Parkinson’s disease are at the very core of a poor, long-term prognosis and they dramatically compound the morbidity of Parkinson’s patients. Therapeutic approaches advocating drug intervention for these comorbid states complicates the disease process further and compromise quality of life as many drugs interact adversely or simply do not work. In our attempt to reduce the total drug burden (TDB) in Parkinson’s disease patients we utilized existing scientific knowledge concerning scientifically based use of bright light therapy for the treatment of insomnia, depression and anxiety. Given the high incidence of these conditions in Parkinson’s disease patients (estimated by some to be as high as 98%), non-drug intervention would be an advantage as it is easy to apply, is non-invasive and uncomplicates the treatment strategy.
Anosognosia is a condition whereby a patient suffering from a CNS disease loses insight into how badly they are debilitated. While the term was originally coined to describe loss of insight in relation to specific disorders such as Alzheimer’s Dementia or stroke (Babinski, 1914), we have observed that Parkinson’s disease patients frequently exhibit various forms of anosognosia. For example, many Parkinson’s disease patients are unaware of the severity or change in severity of primary motor and non-motor symptoms they express, the beneficial effects that a therapy can provide and the adverse effects that some therapies can produce (the classic example is dyskinaesia as many are not aware of how severe their dyskinaesia appears to others, or that they experience it at all). While this may be an advantage in helping the patient to cope with some aspects of their condition it also comes with problems. This being the case, patients with anosognosia are generally intolerant of the delayed therapeutic response and characteristically quit the program early if not reinforced.
Denial is regarded as a defence mechanism and is common in Parkinson’s disease patients. The patient in denial will avoid changing his lifestyle which worsens their Parkinson’s symptoms. They will avoid taking their medication and declare that they will fix it another way or single mindedly engage in activities such as increased physical exercise. This presents a problem not only for stabilizing drug intake but encourages overdosing and noncompliance in the phototherapy program. The combination of anxiety and denial often expedites the progression toward overdosing phenomena.
When a Parkinson’s disease patient is asked to describe how they felt over the past four or more weeks the story is often tainted by the predominating features that the patient experience just prior to attending the session. For example, if a decrease in tremor has been predominant for the first three weeks of the last month but the previous three days was marked by increased tremor then the patient will frequently report deterioration. This is why it important, where possible, to obtain an outside opinion concerning the progression of the patient’s condition. Seeking input from a relative, carer or spouse provides insight into the longer term changes that a patient has experienced and these paint a more objective picture than when a patient is assessed on their own.
There is considerable psychological trauma caused by the presentation of the diagnosis to the Parkinson’s disease patient. They are often severely mentally and physically incapacitated by being informed that they have a debilitating disease for which there is only symptomatic treatment. Given that this can be achieved for only 3-5 years, after which the treatment becomes worse than the disease itself and brain surgery will be likely to be the only relief from the inevitable overdosing phenomena, patients react to a grim, inescapable prognosis. Working to reduce the panic in an effort to obtain compliance is an important part of the program.
Parkinson’s disease patients that are morbidly preoccupied with their disease spend most of their time in self assessment, seeking new treatments, keeping extensive notes or confabulating detailed treatment regimens which contribute to a quicker deterioration. They frantically jump from one novel treatment to another and are susceptible to unproven treatments that have not been shown to be genuinely effective. Equally problematic are the families of many patients in that they can encourage vulnerable patients to pursue a frantic search for the “magic bullet”. These patients either become semi-compliant and resort to thoughtlessly modifying the prescribed drug and random light regimens or leave the program prematurely before a therapeutic effect can be experienced.
There is an ever increasing concern about the amount of drug that Parkinson’s patients are administered to achieve symptomatic control. In addition there are uncountable reports depicting the very serious nature of overdosing with dopamine replacement which produce severe side effects making the treatment “…worse than the disease itself…”. It is interesting to note that in routine clinical practice, basic scientific principles underpinning modern pharmacology are often violated in theory and in practice, with the brain being viewed as a bottomless pit into which we can deposit an endless quantity of dopamine. The classic example is the lack of adherence to principles such as proportional dosing of drug in respect to body weight. Parkinson’s disease patients are routinely treated with the bottom line dose (eg. Levodopa; 100mg tds) with little regard to the patient’s frame, weight or sensitivity to drugs. In spite of endless reports showing that overdosing phenomena such as dyskinaesia, psychosis and symptom exacerbation are a product of dopamine overdosing, the dose of drug routinely administered continues to rise even though the patient’s condition is worsening. In fact, the application of invasive practices such as deep brain stimulation (DBS) are indicated for dose lowering rather than correction of the primary symptoms, per se. Also note that the progressive worsening of the condition is routinely blamed on the advancing disease, even though there are a list of symptoms which are induced or exaggerated by dopamine replacement itself. Such symptoms include:
Dyskinaesia / Hyperkinaesia
Psychosis
Insomnia and Sleep Disturbance
Dopamine Dysregulation Syndrome (DDS)
Choriaform Movement
Freezing (this is really an exaggerate form of bradykinaesia)
On-Off
Dysphonia
Symptom Exacerbation
Compromised Quality of Life
What is of further interest is that while these responses are generally attributed to modified function of dopamine cells in the degenerative state, there is literature suggesting that these events are also a product of dopamine overstimulation in normal cells and not a product of degenerative change (Britt. Med. J. 2, 641, 1973; Mov. Disord 21, 1778, 2006; Lancet, 1,544, 1976; Ann. Neurol. 51, 531, 2002). These clinical finding are grounded in basic scientific work in that dopamine enhancing drugs exert a biphasic effect whereby small doses increase activity while larger doses inhibit it. While this phenomenon has its clinical counterparts, this is of little consequence in the treatment of the disease and accounts for the frequent side effects of continuous, large dose treatment regimens. This is how light therapy becomes so invaluable as a parallel accompaniment to dopamine replacement.
The results from the present work, in our laboratories and in the clinic, suggest that the drug doses necessary to initiate changes in movement are much more subtle than oral dopamine replacement can provide. In addition, the targeting of appropriate dopamine containing sites in the brain that are involved in the development of PD and in the therapeutic effects after oral administration of large doses of drug are virtually impossible to determine. In this regard our discovery that the visual system plays a major functional role in the aetiology of Parkinson’s disease, and is a possible target for treatment, lends new direction and hope for effectively treating the disease.
The parameters of bright light application for treating the depression of Parkinson’s disease were chosen on the basis of several years of scientific work in our clinic and laboratories and on the basis of work with seasonal depression undertaken by renowned researchers such as Professor Michael Terman at The Department of Psychiatry at Columbia University in New York. (See: Terman and Terman, CNS Spectre. 10(8), 647-663, 2005). When we derived a novel drug/light regimen, not only were the secondary symptoms improved in most patients that remained compliant, but their mobility improved and tremor was reduced. In some instances the daily dose of dopamine replacement could be reduced up to 50 % or greater without symptomatic deterioration. This reduced the prospect of adverse reactions compounding over time as doses of dopamine replacement were increased to maintain symptomatic control.
While the treatment is relatively safe and simple to apply it is not something that should be undertaken without professional intervention. While side effects of light overdosing are relatively rare, Parkinson’s disease is renowned for complications due to polypharmacy and overdosing. For this reason professional monitoring of light and drug combinations is essential to ensure that the optimal therapeutic response is achieved and maintained in the long term without light overdosing or drug/light interactions. Light exposure changes the chemistry of the retina and it is by this mechanism that light also changes the chemistry of deep brain. Furthermore, if doses are reduced, this should be done under medical supervision as abrupt withdrawal may cause adverse effects. Also the patient needs an outside, impartial person to make a judgement about their condition who is well versed in the features of the disease, pharmacotherapy and circadian function. Given that any and all of the primary motor and non-motor features of the disease can be expressed to various degrees and in various forms, it is essential that a treatment program is individualized for each patient and this further demands professional intervention and advice.
Our discovery about the benefits of light therapy, as a non-invasive method for altering the function of dopamine cells, not only provides new insight into the biology of the disease but it provides new direction and new hope for many people who have little or no quality of life. Our devoted effort to run the clinic in parallel with our scientific laboratories has enabled us to achieve therapeutic benefits that conventional dopamine replacement therapy has been unable to provide. In the process of providing a new strategy for treating the comprehensive range of symptoms characterizing Parkinson’s disease, we are also providing an evidence-based strategy for reducing the morbidity of this debilitating disorder. There is the added bonus of discovering how other neurological systems, such as the retina, are involved in the process of motor control and in the aetiology and treatment of other neuropsychiatric conditions characterised by nerve cell degeneration.
Our first studies using light therapy in Parkinson’s disease longitudinal, was an open label study published in the refereed journal entitled “Reviews in the Neurosciences 23(2) 199-226, 2012. This long term trial examined the effects of polychromatic (white) light. 156 patients were carefully monitored on 67 variables for more than 4 years while using the light daily. Patients with other neurological disorders including multiple sclerosis, and stroke as well as PD patients that quit the light treatment program early were compared to those that used the legit for the full duration of the
study. The following figures show the effects of daily white light exposure (at the critical time within the light/dark cycle) on bradykinaesia (slowness; Figure 1), tremor (Figure 2), depression (Figure 3), insomnia (Figure 4), anxiety (Figure 5), and nocturnal movement & dyskinaesia (Figure 6).
Motor tests were also used (Figure 7) showing an improvement fine motor control with the floor to knee latency (Figure 8) and the elbow to fist latency (Figure 9) showing significant improvement in the time taken to perform these tasks in those receiving polychromatic light exposure. Reduction in medication (Figure 10) as well as decreased severity of PD over time (Figure 11) was also seen in those patients maintained on light therapy in the long term when used regularly on a daily basis.
Collective examination of these symptoms reveals that PD patients responded well light exposure at the critical time during the Light/Dark cycle. In addition patients using the light on a daily basis did not require as much drug during the course of the study as those that did not.
(Legend for Figures: Open Circles = Regular use of light; Dotted line = intermittent use of light; Closed circles = No light used; Diamonds = other neurological diseases)
With the positive results from our longitudinal study suggesting that the use of white light was beneficial in treating many of the symptom of PD, our next strategy was to undertake a placebo controlled, double blind trial. Such a trial is the golden standard used to determine if a treatment actually works. We completed and then published this trial in September 2018 and it was published in the neurological journal Frontiers in Neurology (Front. Neurol. 9:741. Doi:10.3389/fneur2018.00741). In that study we employed 3 test groups including the active group (polychromatic light; white bars), the first control group (red light; red bars) and a second control group receiving no light (black bars). Examination of the graphs below illustrate that performance on the M.D.S.U.P.D.R.S. scale of assessment for PD improved on the overall score (Figure 1A), on the Part I Score (Figure 1B; non-motor experiences of daily living) and on the Part II Score (Figure 1C; Motor experiences of Daily Living).
The next 2 figures show the performance of PD patients on the PDQ-39 scale which is used to assess the quality of life in PD patients. As seen with the U.P.D.R.S. only those patients maintained on daily polychromatic light showed improvement on the overall score for this assessment (Figure 2A), on the Mobility Scale (Figure 2B) and on the Discomfort Scale (Figure 2C). The patients exposed to Red Light or No Light did not change significantly on these measures during the 2 week course of the study. Similarly, the Emotion Subscale Score (Figure 3A), the Stigma Score (Figure 3B), the Cognition Score (Figure 3C) and the Communication Score (Figure 3D) all improved in Patients treated with white light while the red and no light group did not change significantly on these parameters. Figures 4A through 4C illustrate how depression (Figure 4A), anxiety (Figure 4B) and fatigue, as per the Epworth Sleepiness Scale (ESS: Figure 4C), are improved by exposure to polychromatic light. Note also that the ESS score significantly worsened with red light exposure while the no light group remained unchanged.
**Click on to enlarge
The results from these and other studies (Willis & Turner, Chronobiology International, 224 (3), 521-537, 2007) confirm the therapeutic benefits conveyed by the application of bright light therapy to patients with Parkinson’s disease. These results have been verified in other clinics in Germany (Paus et al, Movement Disorders, 22, 1495-1498, 2007. Doi 10.1002/mds.21542), The Netherlands (Rutten et al, Neurology, 92(11) e1-e12, 1029) and the United states ( Videnovic et al, JAMA, 74(4)411-418, 2017) and form the basis of our ongoing research aimed at refining this approach to better treat PD by less invasive means. More importantly, this research is part of our ongoing investigations examining the role of the retina in the aetiology, progression and treatment of Parkinson’s disease and other neurological disorders. In this regard, it provides a new direction in PD research by defining the circadian system as an integral part of the anatomical complex compromised in this disorder. It also provides a firm basis upon which we can extend relief to PD patients by reducing their dependency on large doses of medication resulting in polypharmacy and adverse side effects. Through this they may experience a better quality of life.
The ends achieved to date suggest that many people thought to be doomed to a life of misery have now been given a second lease on life. In maintaining this objective The Bronowski Clinic and the Institute exists to achieve the following:
To treat the primary motor and non-motor symptoms of depression, anxiety and insomnia using an evidence-based, minimally invasive method that complements dopamine replacement therapy.
To employ a complimentary, minimally invasive treatment that minimizes dopamine replacement strategies so as to avoid their adverse effects.
To decrease the duration of “off-times” between doses of dopamine replacement.
To reduce insomnia and nocturnal movement that occur concomitantly with Parkinson’s disease.
To reduce the total drug burden of Parkinson’s disease patients.
To reduce the incidence of drug interactions.
To reduce the incidence of psychiatric side effects and involuntary movement that long term dopamine replacement produces.
To improve and optimize the quality of life for Parkinson’s disease patients for the duration of their illness.
To slow the progression of the disease.
As a scientifically based philanthropic organization we encourage and appreciate interest in our work as it is our sole aim to improve the quality of life for those who suffer.
A list of our scientific publications and their links to biomedical indexing sources and websites are listed. PDF files of our background publications can also be obtained as well as publications in press, when they become available. These are available upon request.
In 1999 we published work showing that exposure to constant light for 24 hours aided in the recovery from an experimental form of Parkinson’s disease. While this marked the very early stages of our research into seeking ways to slow the degenerative process, it made the discovery that phototherapy might be helpful in treating Parkinson’s disease a possibility. In addition, when we considered that phototherapy had been used for the treatment of depression and insomnia for more than a decade, we decided to set up the Bronowski Clinic to commence treatment with standard light regimens in combination with routine dopamine replacement to treat the depression and insomnia of Parkinson’s disease. At the very least, our goal was to improve these comorbidities using non-invasive techniques that could reduce the number of drugs and the potential threat of developing polypharmacy. With this we were hoping to provide an improvement in the quality of life for these patients. In addition, a growing number of reports intimating that phototherapy might also improve motor function but this was never adequately pursued to a point where its efficacy was determined or the mechanism by which such an effect might occur, was understood. This is where we rearranged our scientific focus to critically analyse the existing literature about the involvement of the visual system and reveal how the retina might be linked to critical deep brain areas to control motor function in health and disease. The more stones we overturned the more we were surprised to find that the visual system, as a component of the circadian system, was intimately involved in the onset, progression and treatment of Parkinson’s disease.
After setting up the Bronowski Clinic specifically for this purpose, phototherapy was administered strategically in line with routine regimens of dopamine replacement and improvement in primary motor and non-motor symptoms occurred. In fact, even at this very preliminary stage it was so effective in some patients that their medication could be reduced by 50% or more without the loss of therapeutic efficacy and the side effects of psychosis and dyskinaesia were dramatically reduced. Substantial improvement in the insomnia and depression which is commonly experienced in Parkinson’s disease was relatively easy to achieve and this was an important finding since drug interactions pose a major problem for Parkinson’s disease sufferers. In short, light therapy provides a means by which many of the symptoms can be treated without complicating their drug treatment and many drug side effects could be avoided. In addition, it is important to point out that the implementation of phototherapy evokes a very subtle change in retinal and deep brain chemistry that, up until now, no one imagined could alter the psychological state and gross behaviour of PD patients. Clearly, the change in chemistry required to repair faulty circuits in disease is much more subtle than previously thought. Consequently, overdosing in Parkinson’s disease with dopamine replacement, antidepressants and soporific drugs can interfere with the efficacy of phototherapy. On this basis, we are working toward greatly reduced and therefore more safe and effective use of drugs in the treatment of Parkinson’s and other neuropsychiatric disorders. This work has broken the chains of tradition in regard to our understanding how our brain works in diseases such as Parkinson’s and takes focus off of dopamine as the sole instigator of the condition.
 In studies in the laboratory which were run in conjunction with the clinic we established a number of principles about Parkinson’s disease which formed the basis of our ongoing research. The first is that there were events in the progress and treatment of the disease that were linked to the circadian (day/ night) cycle suggesting that there was a new anatomy, involving other parts of the brain. To explore how the day/night system was affected, we examined the subparts of this system as it extends from the retina, through the hypothalamus and into the pineal gland might be involved. While we had done much work in the hypothalamus as part of the circadian system the next step was to explore the role of the pineal and the two other primary subparts of the system.
In studies in the laboratory which were run in conjunction with the clinic we established a number of principles about Parkinson’s disease which formed the basis of our ongoing research. The first is that there were events in the progress and treatment of the disease that were linked to the circadian (day/ night) cycle suggesting that there was a new anatomy, involving other parts of the brain. To explore how the day/night system was affected, we examined the subparts of this system as it extends from the retina, through the hypothalamus and into the pineal gland might be involved. While we had done much work in the hypothalamus as part of the circadian system the next step was to explore the role of the pineal and the two other primary subparts of the system.
In a series of ongoing studies we then selectively examined the pineal and the retina on their own merits. In the first work involving the pineal, when we performed a pinealectomy during experimental Parkinson’s disease, recovery occurred more quickly than when the pineal was left intact. Consistent with this, when we blocked the production of melatonin from the pineal by administering constant light the animals recovered significantly quicker. We even employed drugs that blocked the action of melatonin released by the pineal and, for the first time in history, this drug was more effective in recovering animals from Parkinson’s disease than was dopamine replacement alone. In the second phase of the work, we examined the role of the retina since it was the first contact point for light that induced a chemical change in photoreceptors. From previous work on the eye, we knew that dopamine naturally resides there but the fact that retinal dopamine is depleted in the Parkinson’s patient was regarded as being secondary to the dopamine loss in deep brain.
 From this we began to surmise that perhaps the retina played a more important role in the disease itself than was currently believed. In fact, we even hypothesized that the events in the retina might cause, rather than result from Parkinson’s disease itself. To test direct retinal involvement we looked at the effects of anti-Parkinsonian drugs administered directly to the retina itself on experimental Parkinson’s disease. These drugs were injected in doses that were so small that the results could not be explained by the leakage of drug into brain areas involved in motor control or in Parkinson’s disease itself. What we discovered was that anti-Parkinsonian drugs administered to the retina in this way were effective in reversing the symptoms of experimental Parkinson’s disease. This was a major advance in our understanding of the importance of circadian function in Parkinson’s disease in that increasing dopamine during the day was as effective as blocking melatonin during the night. This also served as an explanation as to why anti Parkinsonian drugs become ineffective, and why people develop serious side effects such as insomnia as doses of dopamine replacement are increased over time. The most exciting consequence from these findings is that only very minute doses of dopamine replacement might be needed if the drugs are administered by this route and that the side effects of these drugs might be prevented. This is because the retina is the target for the disease and the stimulation of other dopamine rich areas of the brain, not involved in the Parkinson’s phenomenon, will not be affected with such local injections.
From this we began to surmise that perhaps the retina played a more important role in the disease itself than was currently believed. In fact, we even hypothesized that the events in the retina might cause, rather than result from Parkinson’s disease itself. To test direct retinal involvement we looked at the effects of anti-Parkinsonian drugs administered directly to the retina itself on experimental Parkinson’s disease. These drugs were injected in doses that were so small that the results could not be explained by the leakage of drug into brain areas involved in motor control or in Parkinson’s disease itself. What we discovered was that anti-Parkinsonian drugs administered to the retina in this way were effective in reversing the symptoms of experimental Parkinson’s disease. This was a major advance in our understanding of the importance of circadian function in Parkinson’s disease in that increasing dopamine during the day was as effective as blocking melatonin during the night. This also served as an explanation as to why anti Parkinsonian drugs become ineffective, and why people develop serious side effects such as insomnia as doses of dopamine replacement are increased over time. The most exciting consequence from these findings is that only very minute doses of dopamine replacement might be needed if the drugs are administered by this route and that the side effects of these drugs might be prevented. This is because the retina is the target for the disease and the stimulation of other dopamine rich areas of the brain, not involved in the Parkinson’s phenomenon, will not be affected with such local injections.
We concluded from this research that while dopamine is important in Parkinson’s disease, it does not function on its own. Therefore, treating the disease by replacing dopamine in ever increasing quantities may account for many of the problems that occur. Given that the cells in the substantia nigra are melanocytes whereby melatonin and dopamine live in balance in response to the day night cycle, both have to be taken into consideration if therapeutic outcome is to be optimised.
When we consider the results from our work in the laboratory with the work in the Clinic, it suggests not only that the retina plays an important role in regulating deep brain function, but that we can regulate deep brain function with very subtle changes in chemistry outside the brain. This would not only help us to achieve more effective treatment methods by targeting the sites associated with the brain that are directly involved in Parkinson’s disease, but it permits us to lower the dose of drug required to treat certain conditions where debilitating side effects occur.
This is consistent with our findings in the laboratory where we successfully treat experimental models of Parkinson’s disease with minute quantities of drug but also explains why light treatment is also effective. Our future research is now directed toward optimising the therapeutic benefits of dopamine replacement by involving more subtle manipulation of retinal chemistry that only phototherapy and low dose intraocular methods can provide.